Thermal Properties of Virgin Unfilled PTFE such as Melting Point, Thermal Expansion, Thermal Conductivity, Specific Heat & Heat of Fusion and Thermal stability varies with Molecular weight & crystallanity, thus based on method of fabrication.
The melting point of ‘as polymerised’ PTFE increases with increasing molecular weight and Wunderlich has shown that PTFE also superheats, i.e. the apparent melting point increases with increasing heating rate.
Melting points determined by Differential Scanning Calorimetry* on ‘as polymerised’ powders at a heating rate of 16°C / minute (28.8°F / minute) vary from about 332°C (630°F) for low molecular weight coagulated dispersion polymer to about 346°C (655°F) for high molecular weight granular material. Measurements made at different heating rates indicate that, owing to the superheating effect, these values may be up to 10°C (18°F) higher than would be obtained at infinitely slow heating rates.
The influence of molecular weight on melting point is much reduced after the polymer has been sintered (once melted). Most sintered polymers melt in the range 325330°C (617-626°F) when reheated at 16°C / minute (28.8°F / minute).
The way in which the melting point of sintered PTFE varies with applied pressure was studied by McGeer and Duus who reported the following values:
1 atmosphere 324°C (615°F)
69 atmospheres 335°C (635°F)
207 atmospheres 356°C (673°F)
615 atmospheres 419°C (786°F)
These latter workers used their data to calculate the latent heat of fusion of PTFE as 14 cal / g at 69 atmospheres and 8.4 cal / g at 207 atmospheres. The corresponding entropies of fusion are 0.0240 cal / g deg K and 0.0134 cal / g deg K
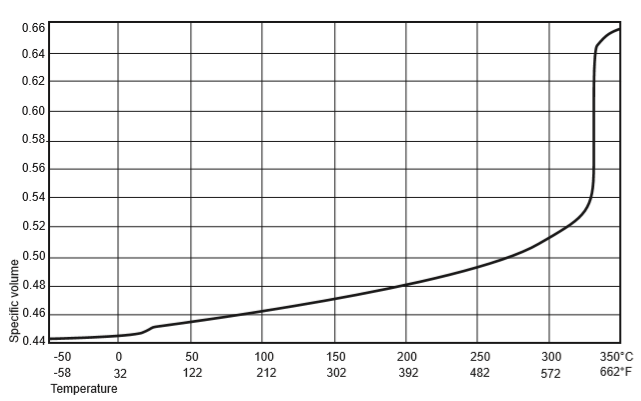
If a graphical representation is made of the specific volume / temperature relationship for highly crystalline fabricated PTFE the form of the graph is shown in Figure 22. This graph clearly reveals the presence of the transition point which occurs at 19°C (66°F) with PTFE. Work done on behalf of AG Fluoropolymers showed that from -60°C to +15°C expansion is approximately linear at 9.5 x 10-5 / °C. Work by Kirby indicates that this coefficient is approximately constant down to -190°C. Above 15°C the coefficient of expansion increases with temperature. Therefore a more useful way of indicating thermal expansion is to express it as a percentage increase in length between two temperatures. Values obtained parallel to and at right angles to the direction of the moulding pressure are quoted separately as they were found to be slightly different.
| Temperature range | Parallel to the direction of moulding pressure |
Perpendicular to the direction of moulding pressure |
|
|---|---|---|---|
| °C | °F | % | % |
| 15 to 30 | 59 to 86 | 0.4 | 0.4 |
| 30 to 50 | 86 to 122 | 0.3 | 0.3 |
| 30 to 100 | 86 to 212 | 0.8 | 0.8 |
| 30 to 150 | 86 to 302 | 1.5 | 1.5 |
| 30 to 200 | 86 to 392 | 2.4 | 2.3 |
| 30 to 250 | 86 to 482 | 3.4 | 3.6 |
Over the temperature range 20-35°C (68-95°F) the thermal conductivity of PTFE is 6 x 10-4 cal / cm s °C. This result may be expressed in a variety of units:
6 x 10-4 cal / cm s °C
2.2 x 10-1 kcal / m h °C
2.6 x10-3 joule / cm s °C
1.7 Btu in / ft2 h °F
Kline measured the thermal conductivity of PTFE at 0, 20, 50 and 70°C (32, 68, 122, 158°F). He reports the conductivity to be fairly constant, with a slight tendency to rise at the higher temperatures. His value is about 5.1 x 10-4 cal / cm s °C.
Eiermann and Hellwege studied this property over a much wider temperature range of -180 to +90°C (292 to +194°F). All their values fell within the range 5.4 to 6.1 x 10-4 cal / cm s °C. It was confirmed that the conductivity tends to rise with temperature though a sharp fall occurred at 20°C (68 F), approximately the temperature at which it has already been noted that a lattice transformation of the crystalline component of the polymer occurs.
^The melting point of a polymer, as measured by DSC, is taken as the temperature at which the peak of the melting endotherm occurs. This peak is reached when the rate of melting is maximal and indicates the melting point of the bulk of the polymer. The final melting point will be slightly higher than this.
The specific heat of PTFE has been determined by Marx and Dole. For temperatures above 40°C(104°F) they give the relationship:
Cp = 0.227 + (2.50 x 10-4) T cal / g °C
The heat capacity, enthalpy and entropy of PTFE have been studied and results are reported in two papers.
Within its normal range of working temperatures, the upper limit of which is generally quoted as 260°C (500°F), PTFE suffers no degradation. Indeed, weight losses observed between 260 and 360°C (500 and 680°F) will be exceedingly small and due to the loss of minute amounts of moisture or gas absorbed in the polymer. At processing temperatures of about 380°C (716°F) the rate of decomposition of PTFE is still very low and it is only at temperatures in excess of 400°C (752°F) that thermal decomposition of pure PTFE becomes significant. Madorsky et al. studied the pyrolysis of PTFE in a vacuum at temperatures from 423.5 to 513°C (794 to 955°F) The decomposition rates which they report at these temperatures are respectively 0.00152% per minute and 1.264% per minute. They further reported that tetrafluoroethylene was virtually the only product of decomposition. This confirmed earlier reports of Lewis and Naylor that when PTFE was decomposed at temperatures between 600 and 700°C (1112 and 1292°F) under pressures of 5 to 760mm Hg the products were C2F4, C3F6 and C4F8 and that the proportion of tetrafluoroethylene among the products increased with decrease in pressure and tetrafluoroethylene was the sole product at very low pressures. Cox et al. have studied the thermal degradation of PTFE with particular reference to the differences observed between degradation in a vacuum and in oxygen. They found that the temperature necessary to achieve a 25% weight loss in two hours was 494°C (921°F) in a vacuum and 482°C (900°F) in oxygen; they concluded, therefore, that the thermal degradation of PTFE was relatively little affected by oxidising conditions. Siegle et al. have evaluated the mechanism of the depolymerisation reaction from research work done on heating thin PTFE films in a vacuum and Jellinek reached similar conclusions. In the case of thicker sections, which are more likely to be met in practice, the rate of pyrolysis is controlled by diffusion of monomer as pointed out by Siegle and Muus. Paciorek et al. studied the auto ignition of PTFE in oxygen and in air. The respective auto-ignition temperatures were 512°C (954°F) and 575°C (1067°F). In oxygen only COF2, CO2 and CF4 were formed, while in air, saturated fluorocarbons, COF2 and CO were the most abundant species.